
Andrey Solver, April, 18, 2025.

Russians have a folk sign, a proverb: “If it snows, it's warm”. For a southern resident, this maxim seems strange. However, it has a deep physical meaning and understanding of nature. The point is in the thermodynamics of the process and in the change in the Gibbs energy, named after the founder of this science.
I have attended lecture courses on chemical thermodynamics and kinetics from many leading universities in the world: MIT, Moscow State University (МГУ), Moscow Institute of Physics and Technology (МФТИ) and, of course, the Institute of Chemistry of St. Petersburg State University (СПбГУ, Leningrad University), the most profound, imho. PhD Ivan Rodionov – I recommend him.
Music: Mikael Tariverdiev, Snow Over Leningrad.
Water vapor in the atmosphere condenses into liquid water or immediately passes into the solid phase (sublimation), and then crystallizes into snowflakes. All these phase transitions are exothermic, that is, they are accompanied by the release of heat into the environment. The latent heat of condensation of water at 0°C is about 2257 kilojoules per kg, the latent heat of sublimation is 2834 kJ/kg, and the heat of crystallization of snowflakes is 334 kJ/kg. The released energy heats the surrounding air by several degrees.
Snow usually falls at -5°C, which is much warmer than frost, say, -20°C, where the air contains 4 times less water. Snow has no source to come from, as the air is dry in frost due to the same thermodynamics, the universal science of transfer and transformation of energy.
It snows on other planets too. On Jupiter, it is made of methane, while on Mars, snow is carbon dioxide, solid CO2. Martian snowflakes are different from ours; they are cubic due to carbon and small, smaller than the thickness of a human hair.
It also snows in the world's oceans. Oceanologists call it "foraminiferal calcite rain". This is a well-established term that describes the process of death of calcite-fixing microorganisms and the sinking of their carbonate shells (tests) to the bottom. More than 50% of the world's ocean floor is covered with these carbonate sediments. Underwater mountain are covered with calcite, CaCO3 is similar to snow-capped peaks on land.
However, strictly speaking, this is not rain, but calcite snow. Foraminifera form their solid skeletons from dissolved CO2, carbonate ions directly by crystallization. This is the main carbon removal process on the Earth. Many have heard the maxim "the ocean absorbs 1/3 of atmospheric CO2", other scientists say 2/3, I recently heard from "reputable researchers" the value of 1/4. Who is right? Nobody.
The world's oceans remove all atmospheric carbon. Because any significant accumulation of carbonate sediments on land is prohibited by chemical thermodynamics: under the influence of fresh water, carbonate minerals dissolve, their dissociation constant, K, is related to pH, hydrogen index or acidity of the medium. Many have probably also heard about "acid rain", a favorite wordmark of environmentalists. Scientifically speaking, any rain is acidic, because a small amount of carbon dioxide from the atmosphere always dissolves in fresh rainwater. When I talk about the impossibility of carbonates forming and accumulating in freshwater on land, of course, I'm talking about geology time, common thermodynamics and chemical kinetics.
The intensity of ocean snow from CaCO3, as well as atmospheric snow from H2O, similarly depends on the thermodynamic conditions of the medium: the degree of supersaturation (mass of the initial substance), temperature, pressure, and Gibbs free energy, the change of which, in fact, creates snowflakes. To start crystallization, it is necessary that the process be thermodynamically beneficial to the system and be accompanied by a decrease in the free energy of the system.
When both snowflakes and calcite foraminifera crystallize, energy is absorbed, and the more energy there is in the system, the more intense the process is. At its high values, snowfall or heavy rain falls from the atmosphere, and "foraminiferal calcite rain" or direct abiotic precipitation of mineral suspension, the so-called "whitening", falls from seawater.
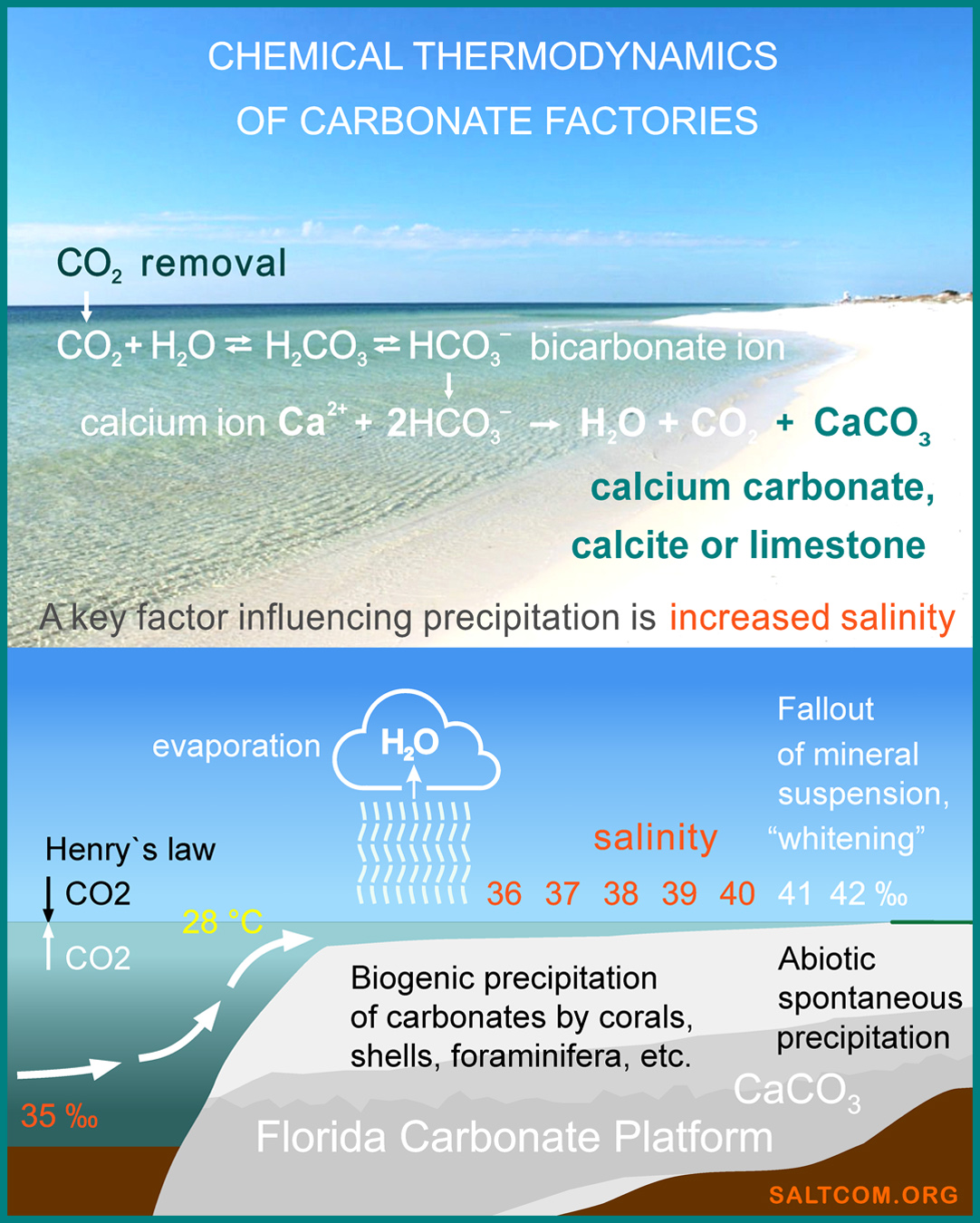
"Whitening" is the process of rapid precipitation of calcium carbonate, CaCO3, most often in the form of aragonite, observed in seawater with increased salinity, for example, on Bahamian banks, off the coast of Florida, in the Red Sea, the Persian Gulf, etc. This phenomenon is associated with special hydrochemical conditions, such as a high degree of supersaturation of carbonate ions, temperature and salinity, which are key parameters for accelerating sedimentation.
The high intensity of heavy rain is a consequence of the large surface energy released from condensing water droplets, the excess of which manifests itself in the form of thunderstorms and electrical discharges. The ocean is also electric — it is an electrolyte, it contains ions of various salts. The value representing the electric field strength in a solution is called the Ionic strength.
![]()
where mi is the molality of ions , zi is their charge. This is the main force responsible for the deposition of carbonates from seawater, and therefore the absorption of carbon dioxide from the atmosphere according to Henry's Law. That is, the mass of carbonate ions in the precipitated mineral is equal to the mass of carbon removed from the atmosphere. The ionic strength drives the global carbon pump.
For seawater with high salinity, it is especially important to calculate accurate ion activity coefficients, which is why we use the Pitzer model. Pitzer's equations provide very accurate modeling of data on the average activity coefficient and equilibrium constants. They are based on rigorous thermodynamics to describe the thermodynamic behavior of ionic solutions using a minimum number of empirical parameters. To understand computing methods, software, and more, I recommend looking at our past researches. From which, for example, it follows that the Calcium Carbonate Precipitation Potential, CCPP (the quantitative indicator of calcium carbonate that needs to be precipitated or dissolved from water to reach equilibrium with CaCO3) does not depend on the degree of dilution of the saline solution.
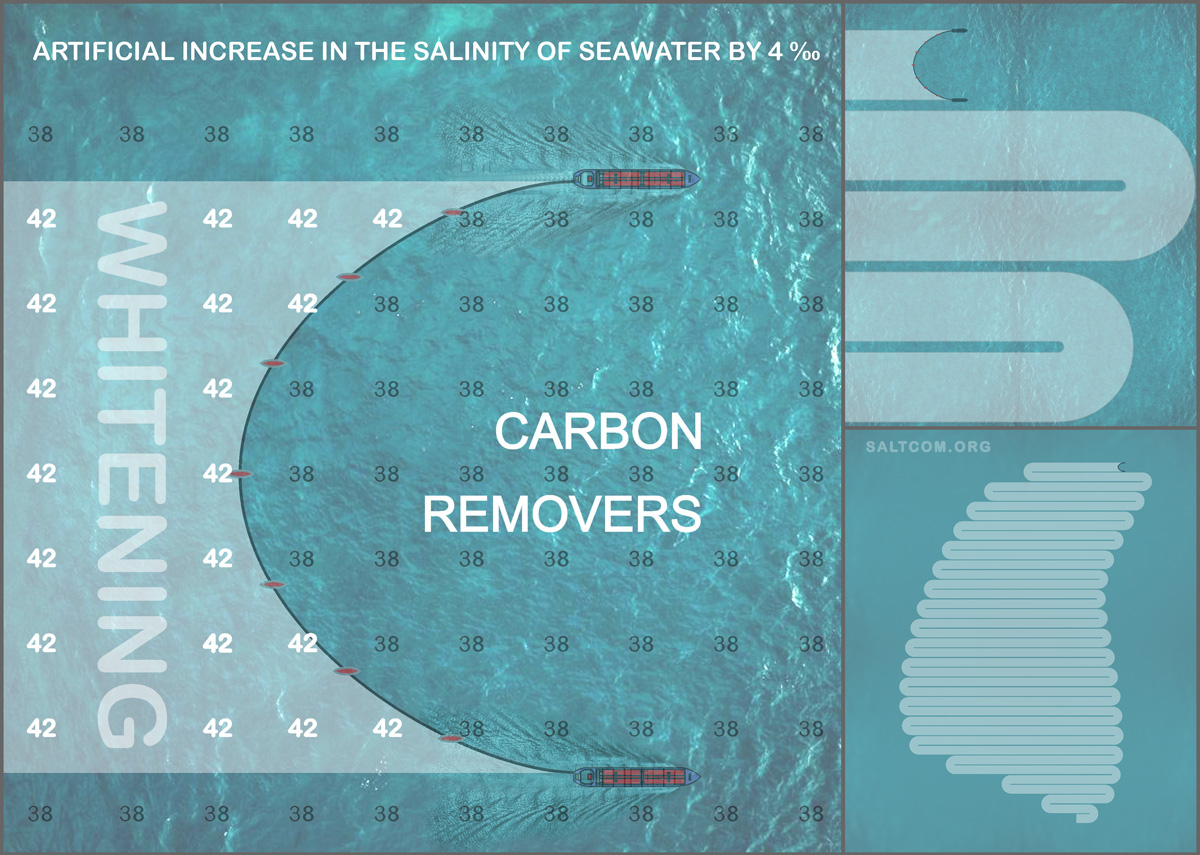
Here I will present simplified calculations of the thermodynamics of CaCO3 deposition using the previously mentioned "Bahamian model" with boundary conditions.:
• Temperature: 28°C (301 K).
• The salinity is artificially increased from 38 to 42 g/l (ΔS = 4 g/l).
• The ocean is an open system with CO2 exchange according to Henry's law.
• Ca2+⁺ and CO32– ions are unlimited (Ca2+⁺ is an order of magnitude larger than carbonate ions and it comes from deep layers).
Henry's Law for CO2 determines carbon dioxid solubility in seawater:
![]()
where:
• PCO2 ≈ 410 μatm = 4.1 ×10-4 atm (atmospheric CO2 concentration in 2025).
• K H ≈ 0.034 mol / (kg ×atm ) at 28°C and salinity ~40 g/L (adjusted using the Pitzer model for ionic strength).
Dissolved CO2 concentration:
CCO2 = 0.034 * 4.1 × 10-4 ≈ 1.394×10-5 mol/kg
Carbonate Equilibrium. CO2 dissolves and participates in equilibria:
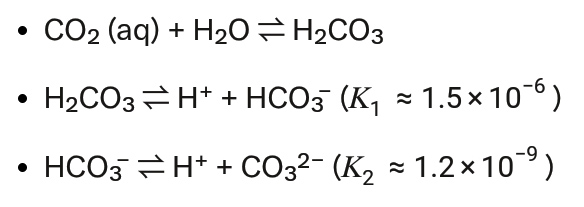
CO2 concentration depends on pH (~8.1 for seawater) and ionic strength. The Pitzer model adjusts equilibrium constants for 42 g/L salinity (ionic strength I ≈ 0.73 mol/kg). At pH 8.1:
[ CO32– ] ≈ 2.3 × 10-4 mol/kg
Ca2+⁺ concentration in seawater (~10.5 mM at 38 g/L) increases proportionally with salinity:
Degree of Supersaturation (Ω)
![]()
where K sp ≈ 3.8 × 10-9 mol2 / kg2 (for aragonite at 28°C).
![]()
Ω > 1, indicating strong supersaturation, which enhances precipitation.
Gibbs Free Energy Change (ΔG):
![]()
where R = 8.314 J/(mol * K), T = 301K.
ΔG = - 8.314 * 301 * ln(702) = - 16,400 J/mol
For 1 ton of CaCO3 (molar mass 100 g/mol, 10,000 mol/ton):
ΔG 1 ton = - 16,400 * 10,000 = - 164 MJ/ton
CaCO3 precipitation grows exponentially with salinity from 35 to 43 g/L. Baseline precipitation at 38 g/L: 0.6 kg/(m2 * year). Assume an exponential dependence:
![]()
where:
• R0 = 0.6 kg/(m2 * year), S0 = 38 g/L
• S0 = 42 g/L
For calibration, use the "whitening" effect (Broecker, Takahashi), where precipitation doubles at 41 g/L (to 1.2 kg/(m2 * year)) and triples at 43 g/L (1.8 kg/(m2 * year)). Fitting yields:
k ≈ 0.17 L/g
Thus:
R(42) = 0.6 * e 0.17 * (42 - 38) ≈ 1.19 kg / (m2 * year)
Precipitation kinetics is described by:
Rate = k r * (Ω - 1) n
where:
• k r ≈ 10-6 mol /(m2 * s) (for aragonite in tropics).
• • n ≈ 2 (empirical reaction order).
• Ω ≈ 702.
Rate = 10-6 * (702 - 1)2 ≈ 0.491mol / (m2 * s)
Convert to kg / (m2 * year):
0.491 * 100 * 3.156×107 ≈ 1.55kg/(m2*year)
However, the empirical exponential model (1.19 kg/(m2*year)) better matches observations. Thus:
R = 1.19 kg / (m2 * year)
For 1 km2 (106 m2);
MCaCO3 = 1.19 * 106 = 1,190 tons/year
CO2 Mass Output
Precipitation of 1 mol CaCO3 releases 1 mol CO2:
Ca2+ + 2HCO3– → CaCO3 + CO2 + H2O
Molar mass of CO2 = 44 g/mol, CaCO3 = 100 g/mol. For 1,190 tons CaCO3 (11,900 mol):
MCO2 = 11,900 * 44 = 523.6 tons/year
Mass Ratios and CO2 Sequestration
Salt Mass: Salinity increase of 4 g/L for a volume of 1 km2 × 4.5 m (V = 4.5 × 106 m3, water density ~1,030 kg/m3, water mass ~4.635 × 10 kg):
Msalt = 4 * 4.635 × 106 = 18,540 tons
Salt / CaCO3 Ratio:
![]()
Salt / CO2 Ratio:
CaCO3 precipitation does not directly sequester CO2 but releases it. Some CO2 may dissolve or be absorbed by biota. Assuming all CO2 remains in the system (conservative approach):
![]()
CO2 Sequestration Over 25 Years;
Energy transition until 2050.
CaCO3 precipitation over 25 years:
MCaCO3, 25 = 1,190 * 25 = 29,750 tons
CO2 release:
MCO2, 25 = 523.6 * 25 = 13,090 tons
Sequestration requires additional mechanisms (e.g., biogenic). In an open system, most CO2 escapes to the atmosphere. Assume zero net sequestration without biological factors.
Economics
Cost of CO2 Removal.
Since the technology of salt carbon sequestration is global, there cannot be a single economic model. If you have watched our work and, in particular, the global picture of Salt of the Earth, you could see in the ranking of salt-producing countries the state of Djibouti, which extracts and supplies about 3.5 million tons of salt per year to foreign markets. This is ten times less than the United States, but still a significant amount. Although not every American will show this country on the map. Salt is extracted there with the most primitive tools on the shore of a salt lake and transported on camels. On the other side of the planet, American robotic complexes extract 2.5 million tons per year in multi-kilometer mines under Lake Erie at a depth of 700 meters.

To assess the economics of the project, it seems logical to me to designate a reference point of $50 per ton. This is the average top price for end-users of salt for deicing urban roads in the United States. The cost of oil, for example, is $575/t in 2025. Despite the seemingly small value, all costs, capital costs, transportation costs, and profits can be placed inside this price. Salt is easy to extract, store and transport. The de-icing market is stagnating, demand is low, environmental issues and other factors that prevent the industry from increasing sales are regularly discussed.
In addition, promising methods of liquid salt mining are simply not available, nobody needs salt brine. While. But if its cost is linked to the ratio of oil or CO2 (burning 1 ton of oil generates 3.15 tons of CO2), it will give impetus to the rapid development of not only the salt industry, but also many related industries. I have already used the word "indulgence" for greenhouse gas producers, now it will become a clearly calculated real compensation, and not vague "carbon credits", which are not popular because they are not secured by anything.
So, for a basic CO2 removal scenario, the cost of salt is:
Csalt = 18,540 * 50 = $927,000
With an annual sequestration of 523.6 tons/year, the price will be:
Cost per 1 ton CO2 = 927,000 / 523.6 ≈ $1,770 / ton CO2 / year
And annually, for the target period of 25 years, the cost of removing 1 ton of CO2 will be:
Cost/ton CO2 = 927,000 / 13,090 = $70.82 / ton CO2

This is the lowest cost in the world. In addition, the technology can be scaled to a global scale, unlike all others.
Any questions by email:
Abyss . Pixar Short Film
I have connected the video for search algorithms and boost Google search results. You don't have to watch it.