1
Kazakhstan`s scientists proposed to salt the Ocean
September 8, 2023 |
||||||||||||||||||||||
2
Introduction
Size of carbon reservoirs on the Earth
|
|||||
1
Kazakhstan`s scientists proposed to salt the Ocean
September 8, 2023 |
||||||||||||||||||||||
2
Introduction
Size of carbon reservoirs on the Earth
|
|||||
|
||||||
| 4
| |||||||||||
5
|
|||||||||
6
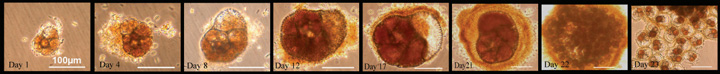
|
||||||||
7
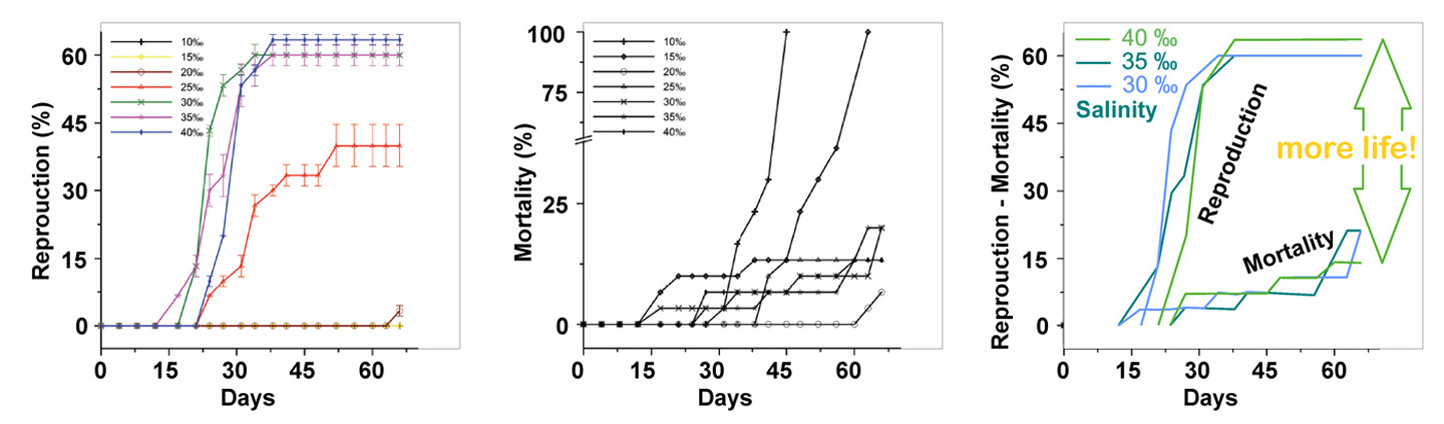
|
||||
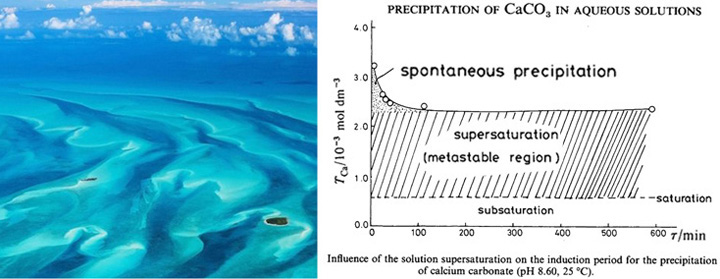
8 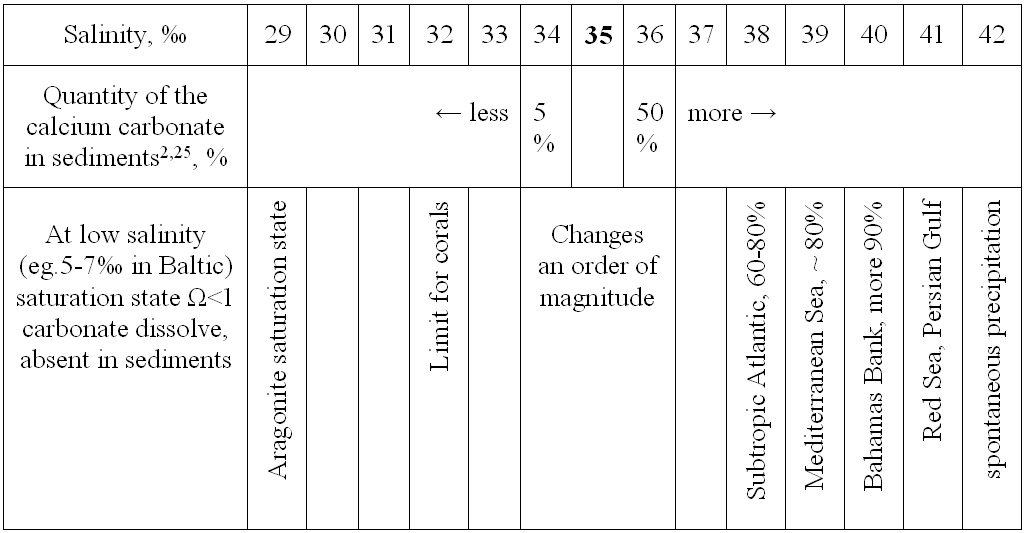
|
|||||
9
|
|||||||||||||||||||||||||||||||||||||
10
|
||||||
11
|
||||
12
|
||||
13
|
|||||||||||||||
14
|
|||
15
|
|||||||||||||||||||||||||||||||||||
16
|
|||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
20
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
21
Reading input data for simulation 2. for Beginning of batch-reaction calculations. EQUILIBRIUM_PHASES: CO2(g), Calcite. CCPP.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||
25
|
||||||||
|
|||||||||||||||||||||||||
27
|
|||||
28
|
|||||
29
|
|||||
30 Welcome to Kazakhstan
|
||||
31
|
||||||||
32
|
||||||
33 Global life hacks
|
||||
34
|
||||||
35
|
|||
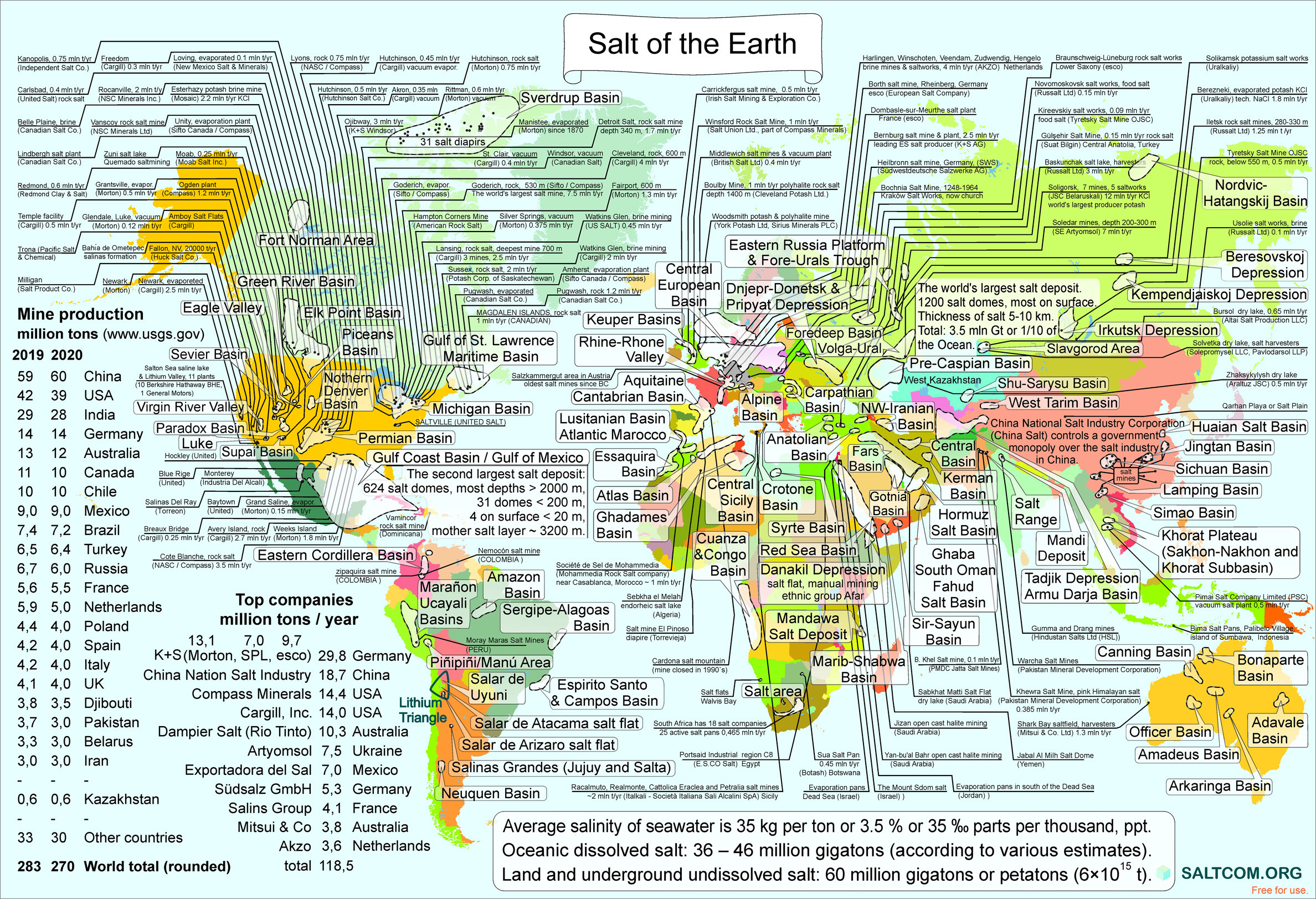 | |||
37 Discussions
|
||||
38
|
|||
39
|
||||
40
The fish kill due to an imbalance of ions and the rapid reproduction of microorganisms during the harmful algal bloom.
|
||||
41
|
||||
42 Vision
|
||||
43
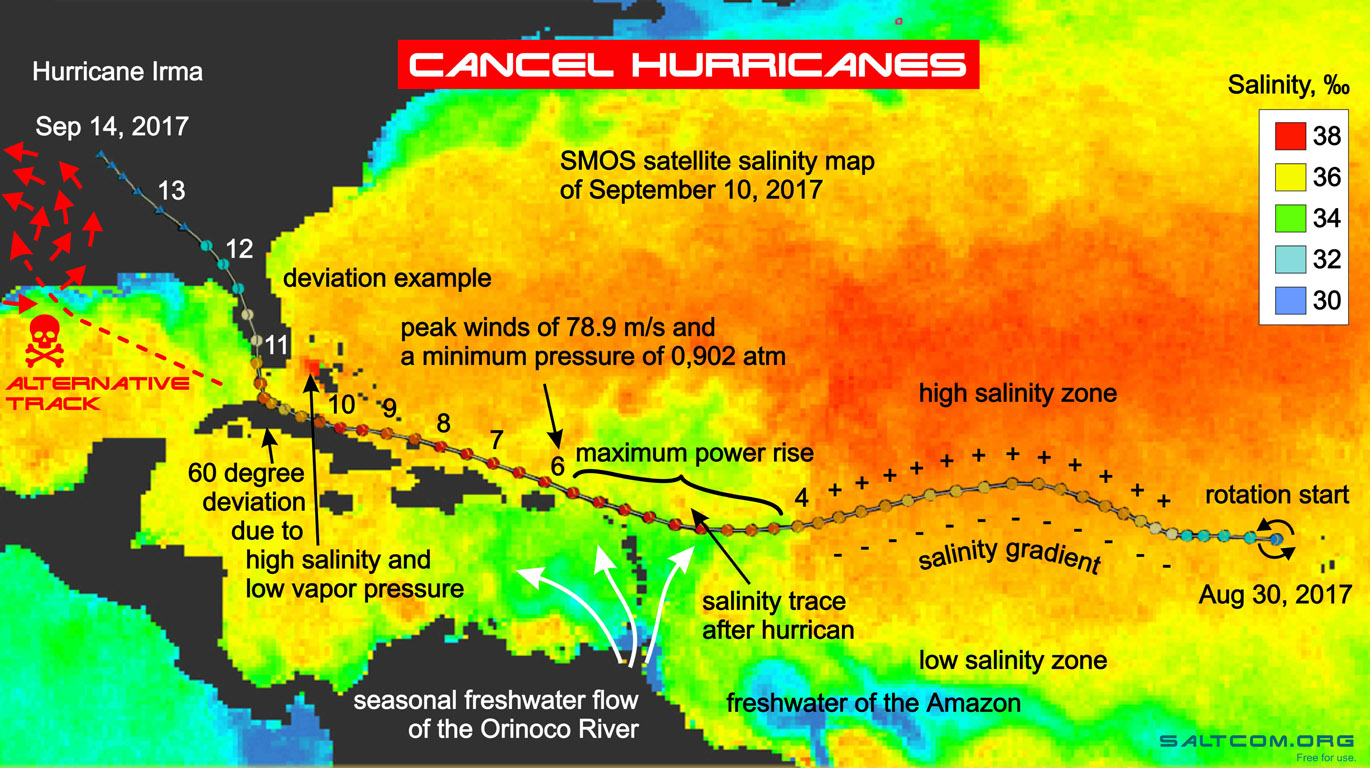
|
||||
44
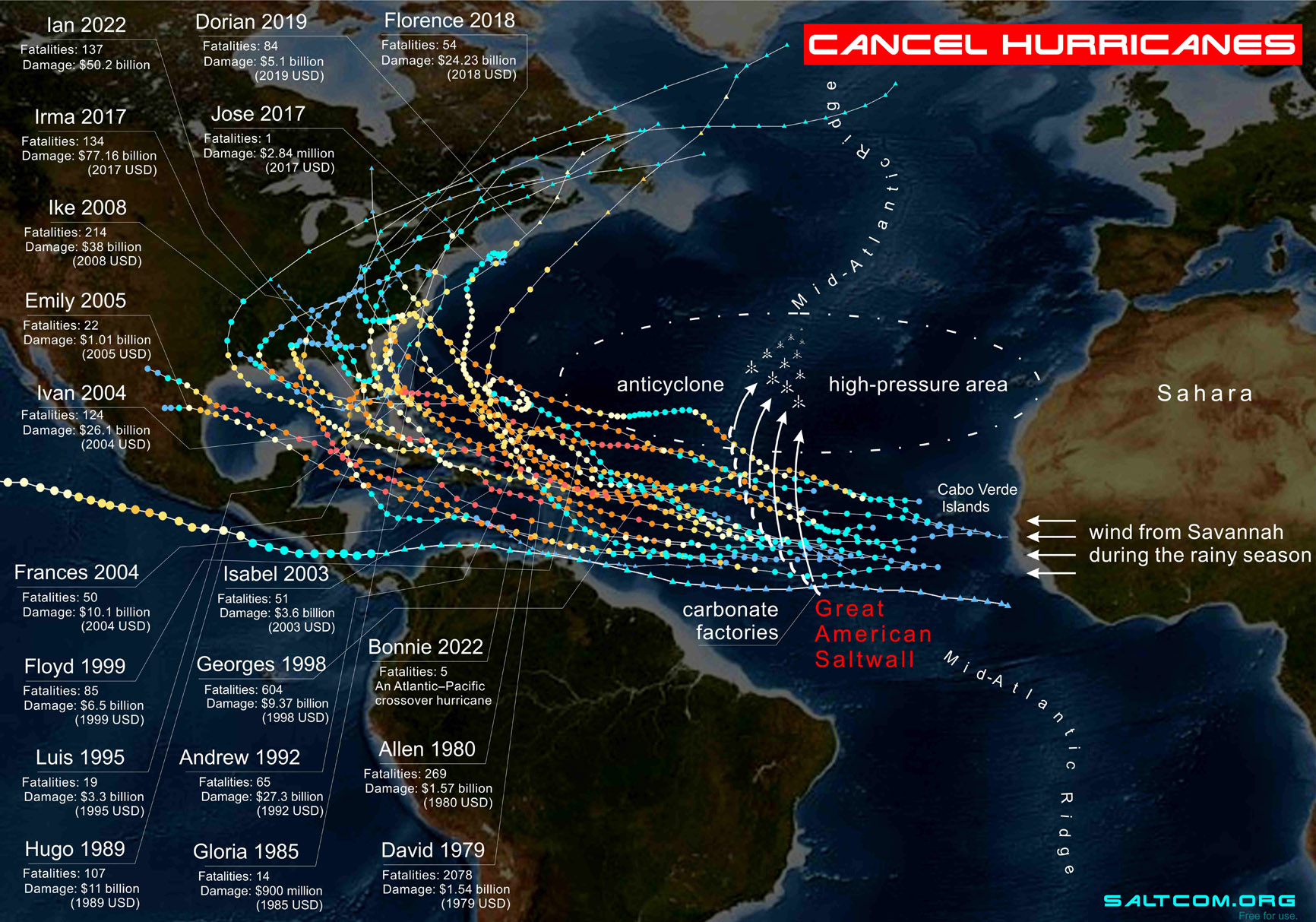
|
||||
45
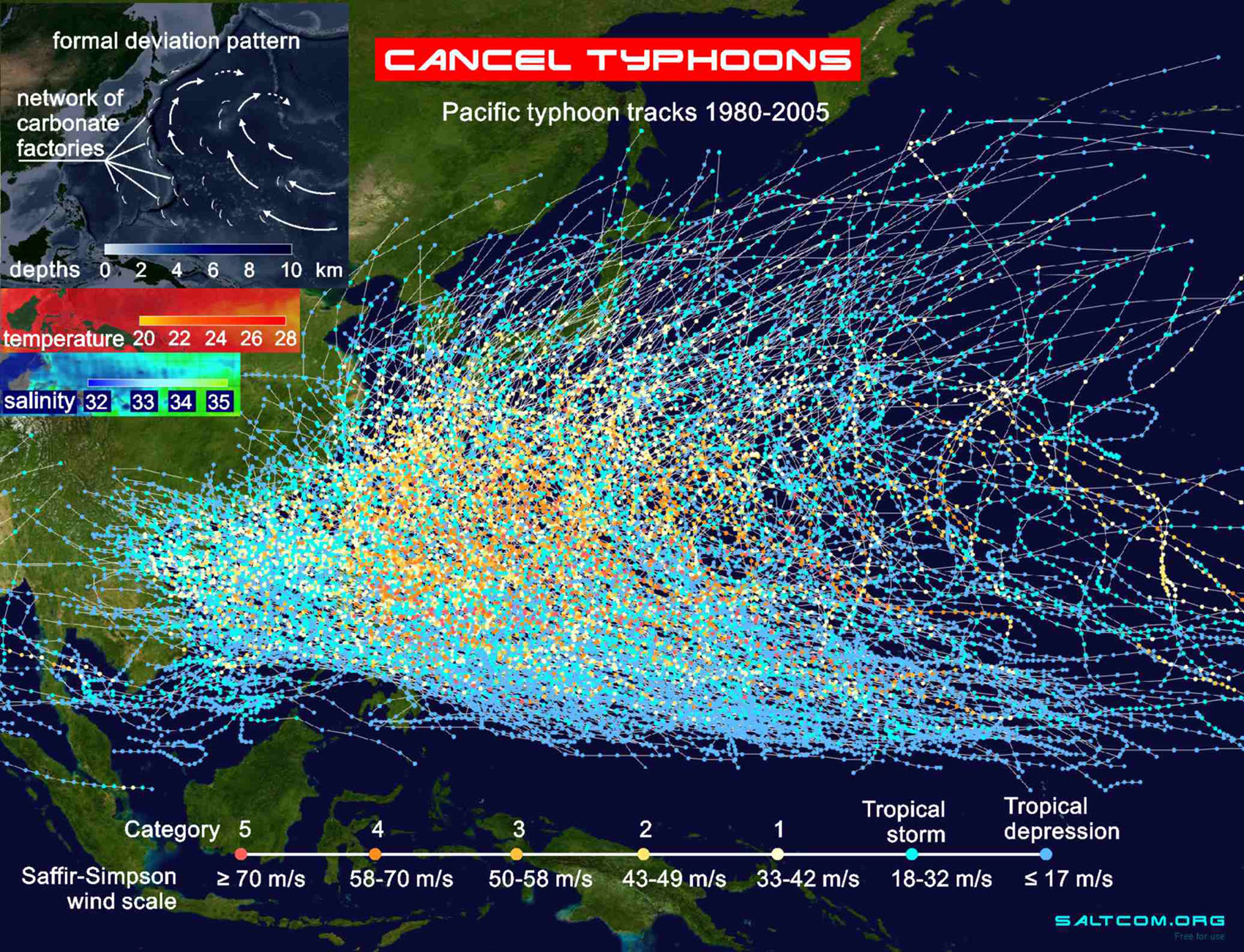
|
||||
46
|
||||
47
|
|||
48
|
|||
49
|
|||
50
|
|||
52
|
|||
53
|
|||
|
|
All texts and images are free to use. You can copy, paste, save, edit, modify, send, receive, repost, accept, transfer, sell, buy, to profit, agree, dispute, develop, ignore, take to heart, do anything with them without restriction. Freeedom!!! |